(1). Calculate the number of molecules in 11.5 dm3 of N2 At STP. practice problem 1.7

Mole ConcepT 01 | How To CalcuLate Number of Moles | Mass Volume Relationship | RevisionПодробнее

Calculate the number of molecules in 1120 ml of hydrogen at STP?Подробнее

How to calculate volume at STPПодробнее

How to Calculate Concentration (from Volume and Moles)Подробнее

How many moles are in 27.0 g of H2O ?Подробнее

Calculating masses in reactions - p27 (Chem)Подробнее

How to calculate number of moles|| chemistryПодробнее

How to Calculate the Number of Molecules in Moles of Carbon... : Chemistry and Physics CalculationsПодробнее

Converting Between Moles and Liters of a Gas at STPПодробнее

Problems From Mole Concept(Plus One )Подробнее

How many molecules of C3H8 are in 50.1 L of gas at STP?Подробнее

Find out the volume of 28.0 g N2 gas at STP | Class Science Important QuestionПодробнее

Calcualte the number of molecules in 5.6 litres of a gas at STP | 10 | MOLE CONCEPT AND STOICH...Подробнее

Solve the Ideal Gas Law for Moles (n)Подробнее

The number of molecules in 22.4 cm3 of nitrogen gas at STP is a. 6.022 x 1020b. 6.022 x 1023c.Подробнее

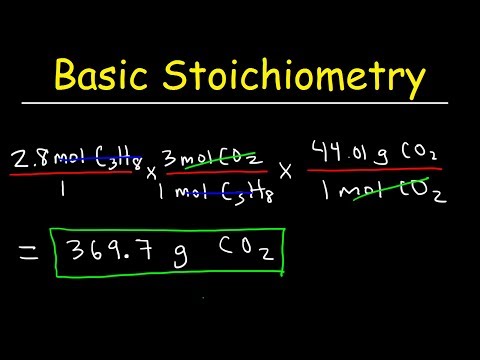
Stoichiometry Basic Introduction, Mole to Mole, Grams to Grams, Mole Ratio Practice ProblemsПодробнее
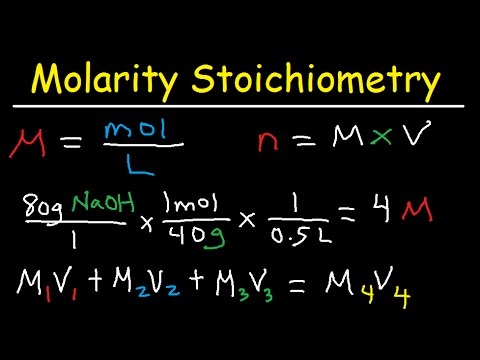
Molarity Dilution Problems Solution Stoichiometry Grams, Moles, Liters Volume Calculations ChemistryПодробнее
How to calculate number of moleculesПодробнее
