#41 - The element rhenium (Re) has two naturally occurring isotopes... Calculate the mass of ¹⁸⁵Re.

Calculate the atomic mass of a hypothetical element "X", if it has 2 naturally occurring isotopes w…Подробнее

Fictional element has two naturally occurring isotopes with the natural abundances shown hereПодробнее

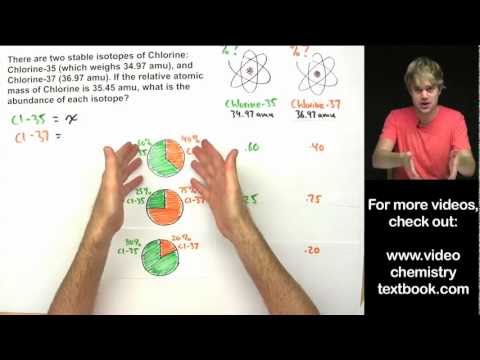
77. Bromine has two naturally occurring isotopes (Br-79 and Br-81) and has an atomic mass of 79.904Подробнее

The element rhenium Re has two naturally occurring isotopes, and with an average atomic mass of 186Подробнее

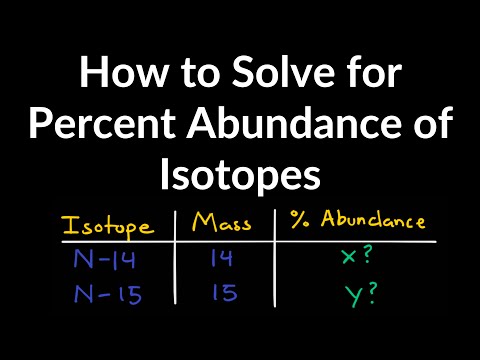
How to Solve for Percent Abundance of Isotopes Examples, Practice Problems, Step by Step ExplanationПодробнее
Bromine has two naturally occurring isotopes Br 79 and Br 81 and an atomic mass of 79 904 amu TheПодробнее

Atomic Mass: How to Calculate Isotope AbundanceПодробнее
An element has two naturally occurring isotopes Isotope 1 has a mass of 120 9038 amu aПодробнее

The atomic mass of copper is 63 546 amu Do any copper isotopes have a mass of 63 546 amuПодробнее

Why are atomic weights not round?Подробнее

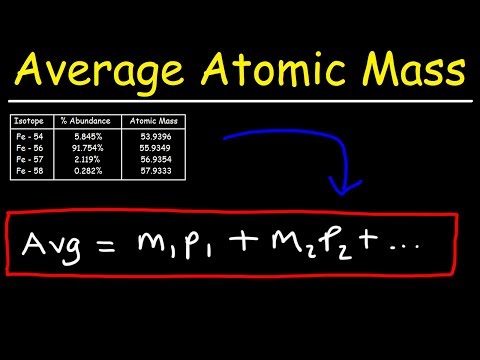
How To Calculate The Average Atomic MassПодробнее
Calculate atomic weight from naturally occurring isotopesПодробнее

Solving atomic mass of unkown isotope - ATOMIC WEIGHTПодробнее

Atomic Masses and the Mass Spectrometer AP Chemistry Problems 3Подробнее

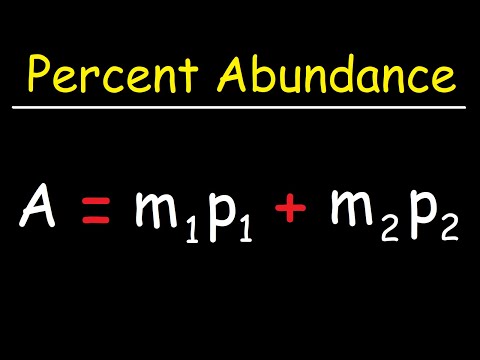
How To Find The Percent Abundance of Each Isotope - ChemistryПодробнее
Average atomic weight - Silver problemПодробнее

The element silicon (Si) has three naturally occurring isotopes: 28Si (atomic mass 27.98 amu), 29Si…Подробнее
