Bromine has two occurring isotopes 79Br with atomic mass 789183 and 81Br with atomic mass 809163

Notation for Isotopes of Bromine (Br)Подробнее

77. Bromine has two naturally occurring isotopes (Br-79 and Br-81) and has an atomic mass of 79.904Подробнее

How to Solve for Percent Abundance of Isotopes Examples, Practice Problems, Step by Step ExplanationПодробнее
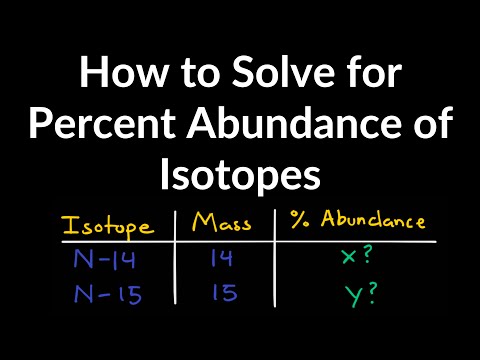
Bromine has two naturally occurring isotopes (Br 79 and Br 81) and an atomic mass of 79.904 amu. Pa…Подробнее

Bromine occurs in nature mainly in the form of two isotopes `._(35)^(79)Br and ._(35)(81)Br`.Подробнее

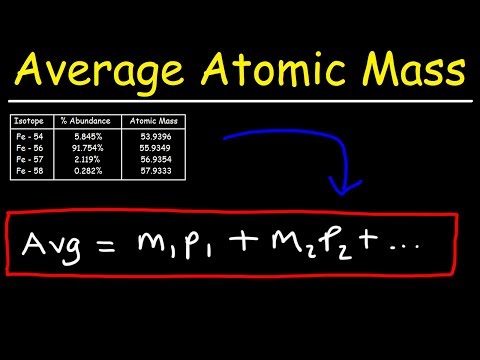
Atomic Mass: How to Calculate Isotope AbundanceПодробнее
How To Calculate The Average Atomic MassПодробнее
Bromine has two naturally occurring isotopes Br 79 and Br 81 and an atomic mass of 79 904 amu TheПодробнее

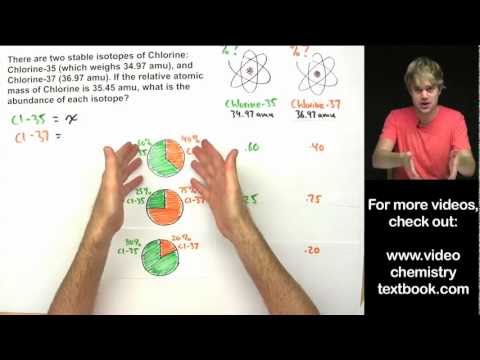
How To Find The Percent Abundance of Each Isotope - ChemistryПодробнее
How small are atoms?Подробнее

An element has two naturally occurring isotopes Isotope 1 has a mass of 120 9038 amu aПодробнее

10. If bromine atom is available in the form of, say, two isotopes 79Br35 (49.7%) and 81Br35 (50.3%)Подробнее

Finding Protons, Electron, Neutrons | Chemistry Class 9 / 10 Science | YouTube Shorts by JP SirПодробнее

What are Isotopes?Подробнее
ISOTOPES , ISOTONES , ISOBARS , ISOMERS / AmaZinG trick! #ShortsПодробнее

GCSE Chemistry - Elements, Isotopes & Relative Atomic Mass #2Подробнее

If bromine atom is available in the form of, say, two isotopes _35^79Br(49.7 %) and _35^81Br(5 O ...Подробнее

How to find average atomic mass of isotopes #shorts #viralПодробнее

Bromine occurs in nature mainly in the form of two isotopes `._(35)^(79)Br and ._(35)(81)Br`.Подробнее
