Calculate relative atomic mass, average atomic weight, percentage abundance of isotope.

MOLE CONCEPT Q #class11 #neet #icse #cbse #likeПодробнее

Isotopes Grade 10 | Part 2Подробнее

CALCULATING % ABUNDANCE FROM RELATIVE ATOMIC MASS (2 ISOTOPES)Подробнее

How to calculate percent abundance from isotopic mass.(new)5070Подробнее

How to Find the Percent Abundance Of IsotopesПодробнее
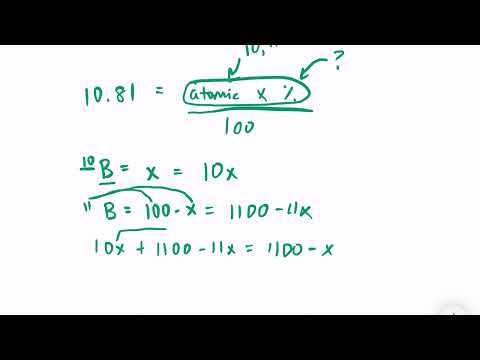
Relative Atomic Mass I WAK Academy | Prof. Wajid Ali Kamboh | Urdu/HindiПодробнее

How to Calculate Atomic Weights using Isotopes l Isotopes #chemistry #isotopes #atomicweightПодробнее

GCSE Chemistry Revision "Relative Atomic Mass"Подробнее

Calculate the Average Atomic Mass of Neon || Practice Problem 1.2 ||Подробнее

ISOTOPES: How to calculate the relative atomic mass of an isotope when given the % abundance?Подробнее

How To Calculate Average Atomic Mass | Chemistry Class 11 | NEET 2023 | Arvind AroraПодробнее

Silicon has three naturally occurring isotopes Si 28, Si 29, and Si 30 The mass and natural abundanПодробнее

Average Atomic Mass | Mole concept | Chemistry | Anupam Gupta IIT Delhi | EmbibeПодробнее

How to calculate percentage abundance of each isotope ?Подробнее
Boron has two isotopes, B-10 and B-11. The average atomic mass of boron is found to be \( 10.80 ...Подробнее

Calculate relative atomic mass from isotopic abundance. Full video at my channel @ChemistryGuruПодробнее

Average Atomic Mass (AAM)| Class 10 Science Term 1 Unit 7 Atoms and MoleculesПодробнее

How to find average atomic mass of isotopes #shorts #viralПодробнее

How to find percentage of Isotopes | Chemistry class 9th |Подробнее
