Consider the oxidation–reduction reaction Al(s)+Ni^2+(a q) →…
4.23a | Complete and balance the following oxidation-reduction reaction: Al(s) + F2(g) →Подробнее

4.19a | Classify this reaction: Na2S(aq) + 2HCl(aq) → 2NaCl(aq) + H2S(g)Подробнее

Oxidation and Reduction ReactionsПодробнее
A satisfying chemical reactionПодробнее

ion electron method || Vishal Rahal || redox reactions || balancingПодробнее

4.20a | Identify the atoms that are oxidized and reduced: Mg(s) + NiCl2(aq) → MgCl2(aq) + Ni(s)Подробнее

17.22b | Calculate the standard cell potential for 3Cu2+(aq) + 2Al(s) → 2Al3+(aq) + 3Cu(s)Подробнее

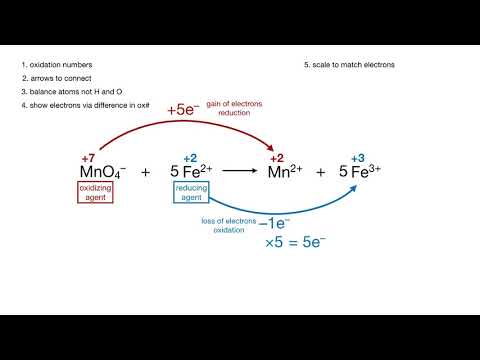
balancing a redox reaction / oxidation number methodПодробнее
Consider the oxidation–reduction reaction Zn(s)+Pb^2+(a q) →…Подробнее

Standard reduction potentials | Redox reactions and electrochemistry | Chemistry | Khan AcademyПодробнее

17.33a | Consider a battery with the overall reaction: Cu(s) + 2Ag+(aq) → 2Ag(s) + Cu2+(aq)Подробнее

Justify that the reaction:2Cu2O (s) + Cu2S (s) → 6Cu (s) + SO2 (g) is a redox reaction...Подробнее

What is Oxidation Reduction & Redox reaction || Diffrence bw Oxidation reaction, Reduction reactinПодробнее

Balancing Redox Reactions Class 11 | Easy Trick to Balance Redox Reaction | Class 11 ChemistryПодробнее

17.11a | Write a cell schematic for Mg(s) + Ni2+(aq) → Mg2+(aq) + Ni(s)Подробнее

4.23c | Complete and balance the following oxidation-reduction reaction: P4(s) + O2(g) →Подробнее

4.24b | Complete and balance the following oxidation-reduction reaction: Ba(s) + HBr(aq) →Подробнее

Calculate the equilibrium constant of the reaction: Cu (s) + 2Ag+ (aq) → Cu2+ (aq) + 2Ag (s)Подробнее

How to write CELL REPRESENTATION (CELL NOTATION)-FOR NEET and JEE👍Подробнее

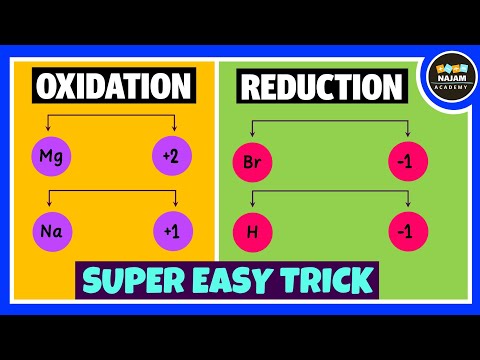
Oxidizing and Reducing Agents | Easy TrickПодробнее
