FDA Cybersecurity and Software Policy Updates: Navigating the New FDA Guidance Documents for MedTech

Complying with FDA Guidance DocumentsПодробнее

New FDA Requirements for Connected Medical Devices. How Cybersecurity Impacts Regulatory ApprovalПодробнее

Medical Device Cybersecurity: Understanding the FDA Guidelines with Mike Kijewski from MedCryptПодробнее

FDA's stance on Off The Shelf vs Custom Software: What you need to knowПодробнее

Navigating the MedTech Cybersecurity EcosystemПодробнее

FDA's Medical Device Cybersecurity GuidanceПодробнее

FDA Finally Released the 2023 Cybersecurity Final Guidance DocumentПодробнее

FDA’s New Guidance on Cybersecurity for Medical DevicesПодробнее

Navigating FDA’s New Cyber Device Paradigm - Cybersecurity & Compliance, Industry Best PracticesПодробнее
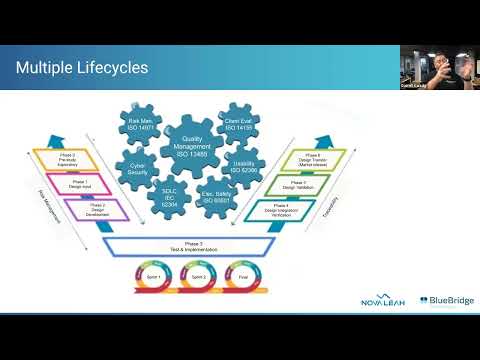
Cloud and Medical Device Cybersecurity: The FDA’s 2023 GuidelinesПодробнее

Securing Healthcare: Navigating FDA Regulations in Medical Device Cybersecurity with Mike NelsonПодробнее

FDA Cybersecurity in Medical DevicesПодробнее

Where to find FDA software and cybersecurity submission requirementsПодробнее

What You Need to Know to Start Implementing the FDA’s New Medical Device Security RequirementsПодробнее

FDA appoints first medical device cybersecurity chiefПодробнее
97 - FDA guidance updatesПодробнее

FDA Cybersecurity Testing Requirements - Interview with Red SentryПодробнее

EPISODE 4: Review and Update of Device Establishment Inspection Processes and StandardsПодробнее
