How many electrons are present in a atom having quantum numbers n=3, l=1?

How many electrons in an atom may have the following quantum numbers? (a) n = 4 ms = – ½Подробнее

How Many Orbitals are in the n=4 shell?Подробнее

How many electrons can fit for the orbital n=3 and l =1Подробнее

In an atom, the total number of electrons having quantum numbers n=4,| m_l|=1 and m_s=-1 / 2 isПодробнее

How many electrons in an atom may have the following quantum numbers i)n-4, ms=-1/2 ii)n=3,l=0Подробнее

What is the maximum numbers of electrons that can be associated with the following set of quantum...Подробнее

How many electrons can fit in the orbital for which n=3 and l=1 ? (a) 2 (b) 6 (c) 10 (d) 14 (NEET...Подробнее

In iron atom, how many electrons have n=3 and l=2? | 11 | ATOMIC STUCTURE | CHEMISTRY | NARENDRA...Подробнее

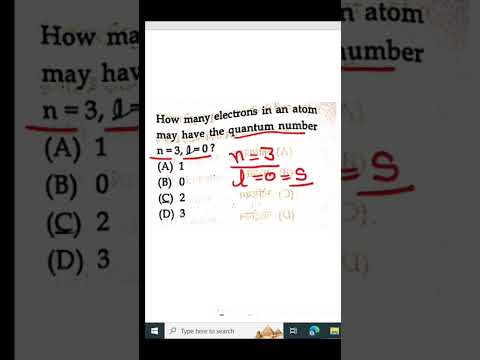
how many electrons in an atom may have the quantum number n=3 , l=0 //Q13Подробнее
Structure of Atom | CBSE Grade 11 Chemistry | Structure of Atom Class 11 Important Questions Part-3Подробнее

, How many electrons in an atom may have the following quantum numbers? (a) n=4, ms=-1/2 (b) n=3...Подробнее

How many electrons in an atom may have the following quantum numbers ? (a) n = 3, m_(s) = -1//2 ...Подробнее

An atomic orbital has n=3, what are the possible values of l and ml? list the quantum numbersПодробнее

Quantum Numbers - How Many Electrons and Orbitals Have the following set of Quantum Numbers?Подробнее
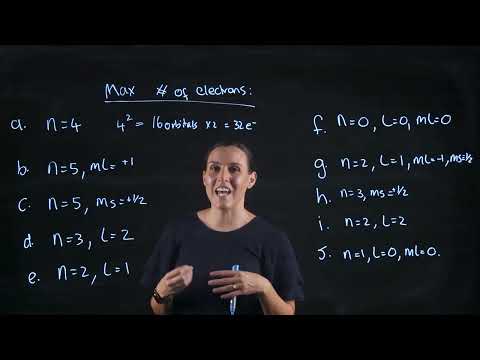
In an atom, the total number of electrons having quantum numbers n=4,| m_ℓ|=1 and m_s=-1/2 is(JEE...Подробнее

How many electrons in an atom may have the following quantum numbers ?Подробнее

, In an atom, for how many electrons, the quantum numbers will be n=3, ℓ=2, m=+2, s=+1/2:- (1) ...Подробнее

What is the maximum number of electrons in an atom that can have the quantum numbers n=3 and l=2 ...Подробнее

The following quantum numbers are possible for how many orbitals? n = 3, l = 2, m = +2 (a) 1Подробнее
