How Many orbitals are there in n=3, the 3rd energy level of an atom?

How many subshells are there in the third principal energy level (n=3) of an atom?Подробнее

How many orbitals in the third shell n =3Подробнее

How Many Orbitals are in the n=4 shell?Подробнее

An atomic orbital has n=3, what are the possible values of l and ml? list the quantum numbersПодробнее

MCQ on Quantum numbersПодробнее

The following quantum numbers are possible for how many orbitals? n = 3, l = 2, m = +2 (a) 1Подробнее

a How many orbitals are there in the third shell n=3? b Show the orbital diagram for nitrogen,Подробнее

For `n = 3` energy level ,haw many orbital of all kinds are possible ?Подробнее

i) An atomic orbital has n=3. What are the possible values of l and m?Подробнее

Using s,p,d,f notations, describe the orbital with the following quantum numbers......Подробнее

Shells, Subshells, and Orbitals - BIOLOGY/CHEMISTRY EP5Подробнее

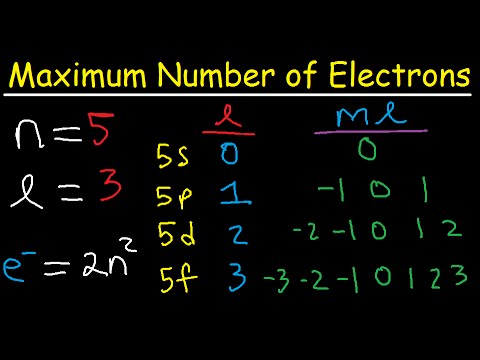
How To Determine The Maximum Number of Electrons Using Allowed Quantum Numbers - 8 CasesПодробнее
For `n = 3` energy level ,haw many orbital of all kinds are possible ?Подробнее

Energy Levels, shells, SubLevels & OrbitalsПодробнее
