How to Best Prepare for an EMA Policy 0070 Submission

Understanding EMA Policy 0070 and Health Canada PRCI Transparency RequirementsПодробнее

Navigating EMA Policy 0070 – How Do We Ensure Compliance?Подробнее

A Streamlined Approach to Policy 70Подробнее

Unlocking the EMA Meetings: the Process and How to Prepare AdequatelyПодробнее

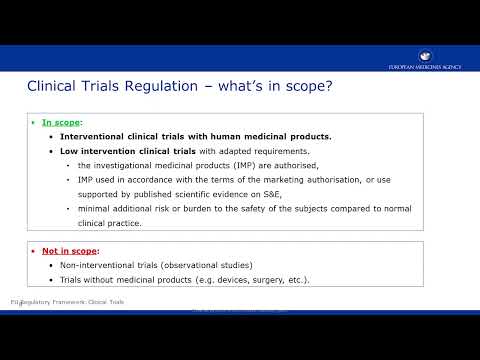
Clinical trials - Regulation EU No 536/2014 and EMA Policy/0070Подробнее

Clinical Data Publication (Policy 0070) webinar - Step 2Подробнее

Reactivation of EMA Policy 0070 Introduction and key recommendations for a successful Publication 20Подробнее

Clinical Data Publication (Policy 0070) re-launch - EMA webinarПодробнее

EU Market Authorisation strategy: Lessons from the first 22 ATMP submitted to the EMAПодробнее

EMA and MHRA Inspections: Successful Planning and Execution Tips and Techniques TrailerПодробнее

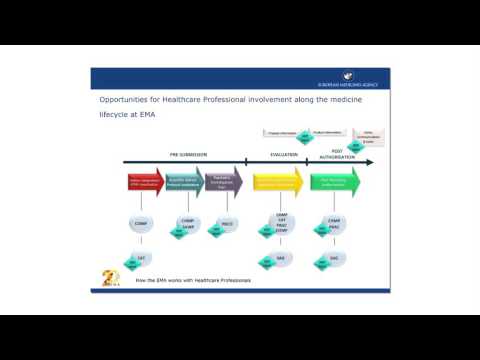
6. How the EMA works with Healthcare ProfessionalsПодробнее
EU Marketing Authorisation | What are the Steps and Timelines for Centralised Procedure at EMA?| DRAПодробнее

Clinical trials in times of Corona - FDA and EMA UpdateПодробнее

Ensuring Success in Implementing EMA Policy 0070 and Health Canada PRCIПодробнее

What is the CHMP?Подробнее

EMA workshop on patient experience data in medicines development and regulatory decision-makingПодробнее

Xtalks eCademy: GCP Training: How to Survive a European Medicines Agency (EMA) AuditПодробнее

EMA Account Management training webinarПодробнее

Overview of the European Medicines Agency (EMA), Part 1 of 3Подробнее