How to Calculate Relative Atomic Mass from Isotopic Composition 1

Mass of isotope. Part 1.Подробнее

Isotopes Grade 10 | Part 2Подробнее

Calculating Relative Atomic Mass from Isotopic Composition | Year 11 Chemistry Module 1Подробнее

How to calculate percentage abundance of each isotope ?Подробнее
Question 3 on How to Calculate Relative Atomic Mass from Isotopic CompositionПодробнее

Calculate Relative Atomic Mass from Isotopic AbundanceПодробнее

ISOTOPES: How to calculate the relative atomic mass of an isotope when given the % abundance?Подробнее

Chemistry A level Relative atomic mass difficult question solved pass your exams @letsgettothemarksПодробнее

GCSE Chemistry Revision "Relative Atomic Mass"Подробнее

Relative Atomic Mass & Abundancy of IsotopesПодробнее

Boron has two isotopes, B-10 and B-11. The average atomic mass of boron is found to be \( 10.80 ...Подробнее

RELATIVE MOLECULAR MASS :PART 1 [JAMB UTME, WAEC, NECO]Подробнее
![RELATIVE MOLECULAR MASS :PART 1 [JAMB UTME, WAEC, NECO]](https://img.youtube.com/vi/FJ1S8NqXvpo/0.jpg)
Isotopy and Isotopes|Calculation of Relative Atomic MassПодробнее

Isotopy | Calculations on Isotopes | Chemistry TutorialПодробнее

Question 2 on How to Calculate Relative Atomic Mass from Isotopic Composition #chemistryПодробнее

The Atom & Isotopes // Preliminary HSC ChemistryПодробнее

Atomic Number, Mass Number, and Net Electric ChargeПодробнее
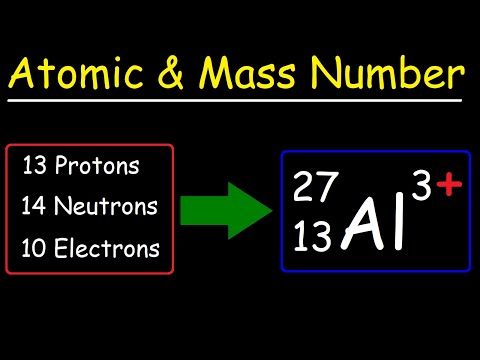
1. Lecture 1-Isotope notation, average atomic mass, empirical formula (mass & percentage)Подробнее

Chlorine has two isotopes Cl35 and Cl37. Ratio present in nature is 3:1. Calculate the avg atomic wtПодробнее
