How to Convert Moles of CaCO3 to Grams

CaCO3 contains 3 gram carbon. The mole of CaCO3 are:Подробнее

How To Convert Moles to GramsПодробнее
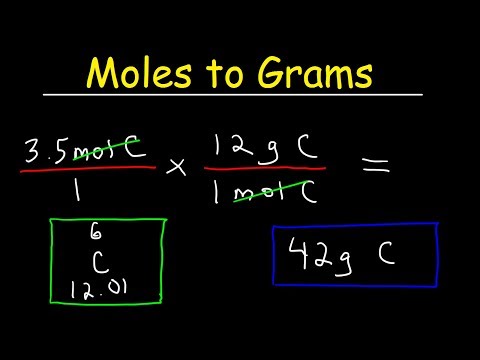
Moles to Grams in One minuteПодробнее

Q3. Calculate the mass of 5 moles of CaCO3 in gramsПодробнее

Converting Grams of CaCO3 to Atoms of OПодробнее

How to Convert Grams to Moles ; (Mole Conversions Made Easy)Подробнее

How to Convert Moles of Ca3(PO4)2 to GramsПодробнее

How to Convert Moles of LiCl to GramsПодробнее

Converting Between Grams and MolesПодробнее

Molar Mass / Molecular Weight of CaCO3: Calcium carbonateПодробнее

Mole Conversions Made Easy: How to Convert Between Grams and MolesПодробнее

Sulfuric Acid on Toilet Paper Spawns a DemonПодробнее

How many grams of carbon dioxide can be produces by Decomposing 6.5 moles of CaCO3 Practice Prob 1.5Подробнее

unexpected neet result #physicswallah #yakeen2.0 #competitionwallahПодробнее
sulphuric acid #shortsПодробнее

Finding Grams of Ca from grams of CaCO3Подробнее

How to Convert Moles to GramsПодробнее

How to Convert Moles of CaCl2 to GramsПодробнее

How many moles of calcium carbonate `(CaCO_(3))` are present in `10 g` of the substance ?Подробнее

S1.4.3 Calculating mass in grams from amount (in mol)Подробнее
