Key differences between 510(k) and PMA pathways at the FDA

FDA Regulation of Medical Devices and Software/AppsПодробнее
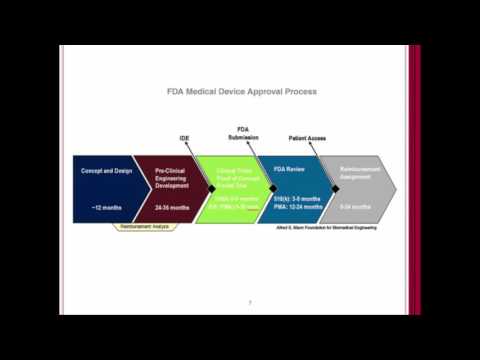
Concerns about 510(k) ProcessПодробнее

Premarket Approval (PMA) Agreement with FDA for the Medical Device CompaniesПодробнее

Medical Device Regulations / FDA ApprovalПодробнее

Understanding the Difference Between FDA Approval and FDA ClearanceПодробнее

Video Briefs - 510(k) vs. PMA Regulatory Strategy - Approaching FDAПодробнее

What is the difference between a 510k and De Novo?Подробнее

How to register a Medical Device with FDA? (510k, PMA, de Novo...)Подробнее
Comparing Usage of FDA 510(k) and Premarket Approval Pathways within Orthopaedics to Other...Подробнее

Perclose: 510(k) vs. PMAПодробнее

Choosing the Best FDA Submission Pathway for your ProductПодробнее

U.S. FDA’s 510(k), IDE, and PMA Documentation, Submission and Approval ProcessПодробнее

Understanding the FDA Medical Device 510k ProcessПодробнее

US FDA 510(k) Clearance & Premarket approval-Operon StrategistПодробнее

How to Prepare a Medical Device 510k Submission for FDAПодробнее

The 3 Types of 510(k) SubmissionsПодробнее

Basics of 510(k) Clearance ProcessПодробнее

How to Save Costs on Your 510(k) or PMAПодробнее

What do FDA reviewers look for in 510 (k) and IDE submissions?Подробнее

Premarket Approval PMA Agreement with FDA for the Medical Device CompaniesПодробнее

Investigational Device ExemptionПодробнее
