PDSVISION | Medical Devices Risk Management ISO14971 in Codebeamer

ISO 14971:2019 – Risk Management for Medical Devices part 1Подробнее

Medical Device Compliance with IEC 62304 and ISO 14971Подробнее

Intland Webinar - QA & Testing in Medical DevicesПодробнее

ISO 14971: Medical Risk Management Best PracticesПодробнее

Medical Device Development: Quality Assurance and TestingПодробнее

Risk Management in Medical Device DevelopmentПодробнее
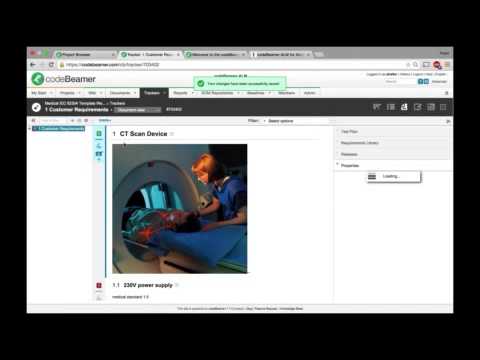
Setting up Medical Device Software Development Projects in Compliance with IEC 62304 and ISO 14971Подробнее

Risk management for medical devices and ISO 14971 - Online introductory courseПодробнее

How to work with medical device risk managementПодробнее

ISO 14971 Applied Medical Device Risk Management FULL Course [Part 1]Подробнее
![ISO 14971 Applied Medical Device Risk Management FULL Course [Part 1]](https://img.youtube.com/vi/8qtjQNYFwfU/0.jpg)
MDR Risk Management training course - Build, document & maintain an ISO 14971:2019-compliant systemПодробнее

What is the best way to perform risk management?Подробнее

Medical Devices - ISO 14971 : Risk ManagementПодробнее

How to estimate risk for a medical device according to ISO 14971:2019Подробнее

ISO 14971:2019 & TR 24971 Explained - Medical Device Risk ManagementПодробнее

ISO 14971 and the risk management of medical devicesПодробнее

ISO 14971 (Medical devices: Application of risk management to medical devices)Подробнее
