The pH of a solution is 3.301.what is the hydrogen ion concentration of the solution ?

How to Calculate Hydrogen Ion Concentration from pHПодробнее

The pH of the solution is 4. The hydrogen ion concentration of the solution in mol/litre is: (a) ...Подробнее

pH and Hydrogen Ion ConcentrationПодробнее

Buffer solution | Ionic Equilibrium class 11thПодробнее

the pH of a solution is 5 if its hydrogen ion concentration is decreased by 100 times then natureПодробнее

Hydrogen Ion Concentration (Part 3): Determination pH of an Unknown SolutionПодробнее

Determine PH of a solution given the hydrogen concentrationПодробнее

Calculating pH From Hydronium Ion ConcentrationПодробнее

If the hydrogen ion concentration of a given solution is 5.5 xx 10^(-3) mol litre^(-1), the pH o...Подробнее

You have two solutions, A and B. The pH of solution A is 6 and pH of solution B is 8. Which solutionПодробнее

pH Potential of Hydrogen | Important points about pH | Learno Spark - Biology #phvalueПодробнее

Calculate the Hydrogen Ion ConcentrationПодробнее

The pH of the solution is 4. The hydrogen ion concentration of the solution in mol/litre is | C...Подробнее

What is the pH of a solution whose hydrogen ion concentration is 3.2 x 10-4 M?Подробнее

The hydrogen ion concentration of the ocean is about 2 × 10^-9 M. what is the pH?Подробнее

pH, pOH, H3O+, OH-, Kw, Ka, Kb, pKa, and pKb Basic Calculations -Acids and Bases Chemistry ProblemsПодробнее
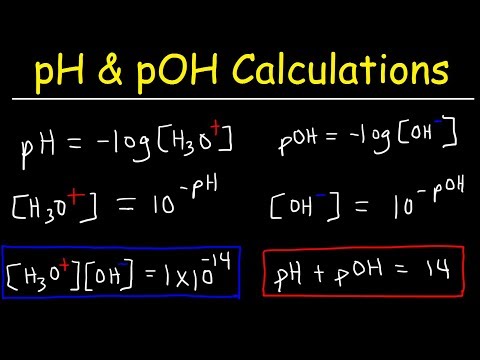
What is the pH of water?Подробнее

The pH of a solution is 9.67. Calculate the hydrogen ion concentration in the solution. [H+] = 2.1 …Подробнее
![The pH of a solution is 9.67. Calculate the hydrogen ion concentration in the solution. [H+] = 2.1 …](https://img.youtube.com/vi/HEOFS2_yJak/0.jpg)
Calculate the hydrogen ion concentration and the pH of each of the following solutions of strong ac…Подробнее
