Understanding the Investigational Device Exemption (IDE) Process

Understanding the FDA Pre-Market Approval (PMA) Process for Medical DevicesПодробнее

What Is An Investigational Device Exemption (IDE)?Подробнее

Explaining Neuralink’s Human Trials ProcessПодробнее

How to Register a Medical Device in the USA (IDE)Подробнее

Investigational Device ExemptionПодробнее

US FDA Investigational Device Exemption (IDE) OverviewПодробнее

"Strategy and Preparation of an Investigational Device Exemption (IDE) Application"Подробнее

FDA Regulation of Medical Devices and Software/AppsПодробнее
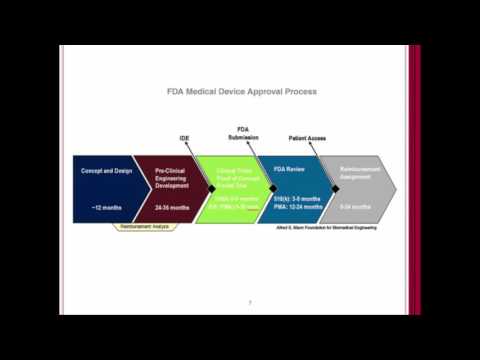
Webinar: Introduction to US FDA Medical Device Regulations (510k, De Novo, IDE, CAPA, eMDR)Подробнее
