Using the Rydberg Equation to Solve Transitions Between Energy Levels

Rydberg equation in 2 minutesПодробнее

Bohr Atomic Model, Rydberg's Equation, Hydrogen Spectrum // HSC PhysicsПодробнее

Introduction to the Bohr Model of Hydrogen Atom, Including Spectroscopic CalculationsПодробнее

Atomic SpectraПодробнее

Quantum Physics - Transitions between energy levelsПодробнее

2.1 PART 3 - The Rydberg EquationПодробнее

What is the wavelength of light emitted when the electron in a hydrogen atom undergoes transitionПодробнее

Schrodinger equation solutions to the hydrogen atomПодробнее
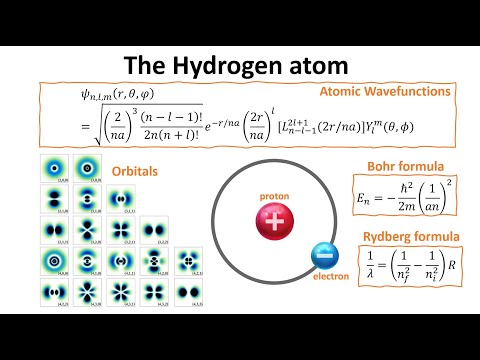
S1.3.2 The hydrogen emission spectrumПодробнее

Lecture 4.2 Bohr Model of the AtomПодробнее

Using Rydberg's FormulaПодробнее

Week 4 Chemistry Bohr’s formula & Rydberg equation Lesson 2.1e SDSПодробнее

PP07 1QuantumTheoryAПодробнее

How to Calculate Energy Level Transitions for the Hydrogen Atom with Electron Excitation/RelaxationПодробнее

FSc Chemistry Book1, CH 5, LEC 12: Hydrogen SpectrumПодробнее

Calculate the wavelength of the photon emitted when the hydrogen atom transition from n=5 to n=3.Подробнее

27: Using the Rydberg equationПодробнее

Quantum Model of the AtomПодробнее

Bohr Model of the Hydrogen Atom, Electron Transitions, Atomic Energy Levels, Lyman & Balmer SeriesПодробнее
