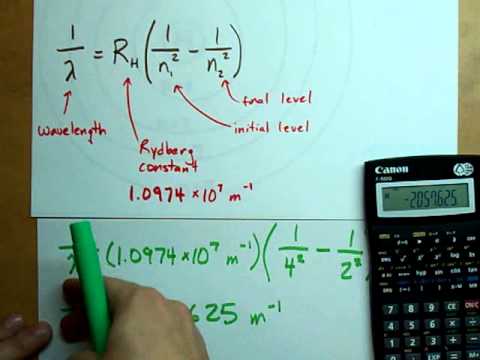
Wavelength of Light Released from Hydrogen
Terrence Howard: "Every human being needs to know this"Подробнее
What is the wavelength of light emitted when the electron in a hydrogen atom undergoes transitionПодробнее

Calculate the wavelength, in nanometers,of the spectral line produced when an electron in a hydrogenПодробнее

Rydberg Constant | Hydrogen Spectra | Viva VoceПодробнее

What is the wavelength of light emitted when the electron in a hydrogen atom undergoes transition..Подробнее

Determination of Wavelength & Dispersive Power | Diffraction Grating | Practical FileПодробнее

How to Solve for Wavelength of Light Absorbed or Released Example, Practice Problems, CalculationПодробнее

Huygens principle and Wavefront || Animated Hindi explanation ||Wave optics || 12th class || physicsПодробнее

Coherence part 3: This is not a wave.Подробнее

What is the wavelength of light emitted when the electron in a hydrogen atom undergoes transition...Подробнее

CHEMISTRY 101: Electron Transitions in a Hydrogen AtomПодробнее

CHM1011 & CHM1051 - The Rydberg EquationПодробнее

Bohr Model of the Hydrogen Atom, Electron Transitions, Atomic Energy Levels, Lyman & Balmer SeriesПодробнее

Energy Released: ΔE for electron in Hydrogen AtomПодробнее

Calculate the wavelength of the photon emitted when the hydrogen atom transition from n=5 to n=3.Подробнее

Energy from Wavelength: Electromagnetic Radiation CalculationПодробнее

⚗️ Wavelength of Light for a Transition in the Hydrogen Atom (Question 1)Подробнее

Atomic Structure - Finding the wavelength of light to excite a Hydrogen electronПодробнее

Calculate the Energy, frequency & wavelength of an electron transition in the Bohr Atom.Подробнее
