Webinar for Special 510(k) Submissions
510(k) Tip - What's 'special' about a Special 510(k)?Подробнее

Webinar: Medical Devices Cybersecurity in 510(k) Premarket SubmissionsПодробнее

Using Clinical Data in your 510(k) FDA eSTAR - webinar Feb. 8, 2024Подробнее

510(k) Submission Predicate Selection Webinar - Updated for 2022Подробнее

510(k) Project Management - Updated for 2021Подробнее

SARACA Free Live Webinar on Roadblocks in the 510(k) Submission ProcessПодробнее

Using the new eSTAR templates for a 510(k) submission and the FDA eSTAR draft guidanceПодробнее

510(k) eSTAR Webinar - Indications for Use and ClassificationПодробнее

The “New” 510(k): How Do You Show Substantial Equivalence without Using a Predicate?Подробнее

Mastering your 510(k) submission processПодробнее

How to Prepare a Medical Device 510k Submission for FDA | Rob Packard | Joe Hage | UpdatedПодробнее
Boiler plate documents for the 510k Webinar- Sections 3, 6, 7, and 8.Подробнее

Software Validation Documentation for FDA 510(k) pre-market notification submissionПодробнее

Webinar: Sterility Information in Premarket Notification (510k) Submissions for Medical DeviceПодробнее

How to prepare an FDA eSTAR 510(k) submissionПодробнее

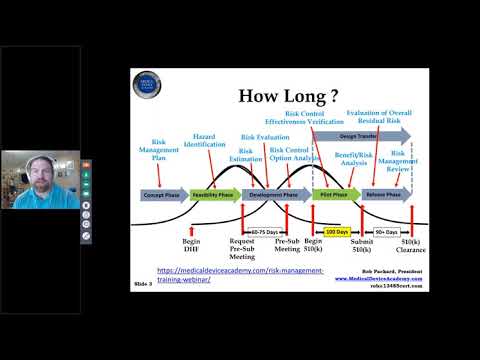
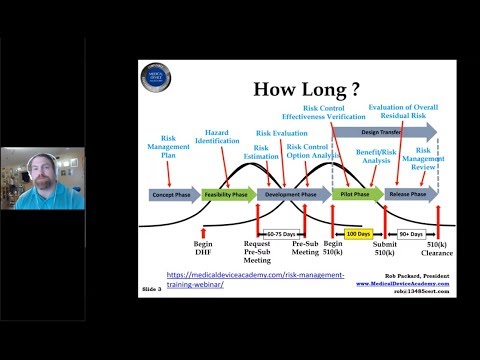
510(k) Pre-Submission Webinar - Stop Wasting Time and Request a Pre-Sub MeetingПодробнее
How to Prepare a Medical Device 510k Submission for FDA | Rob Packard | Joe HageПодробнее
U.S. FDA’s 510(k), IDE, and PMA Documentation, Submission and Approval ProcessПодробнее

Labeling and UDI Requirements for a 510(k) SubmissionПодробнее

510(k) Frequently Asked QuestionsПодробнее
