Cybersecurity Documentation for a 510(k) Submission

WI-007 Cybersecurity Requirements WebinarПодробнее

Where to find FDA software and cybersecurity submission requirementsПодробнее

Using Clinical Data in your 510(k) FDA eSTAR - webinar Feb. 8, 2024Подробнее

Webinar: Medical Devices Cybersecurity in 510(k) Premarket SubmissionsПодробнее

De Novo vs 510k - What’s the differenceПодробнее

510(k) Project Management Best PracticesПодробнее
How much does a 510(k) cost? - FY 2024Подробнее

FDA Cybersecurity Testing Requirements - Interview with Red SentryПодробнее

How to Prepare a Medical Device 510k Submission for FDAПодробнее

FDA Cybersecurity and Software Policy Updates: Navigating the New FDA Guidance Documents for MedTechПодробнее

How to Prepare a Medical Device 510k Submission for FDA | Rob Packard | Joe Hage | UpdatedПодробнее
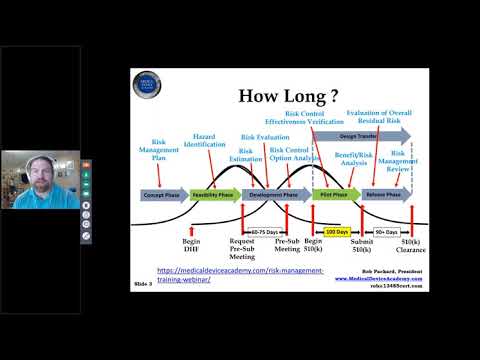
Cybersecurity Webinar - Learn what the FDA wants in your 510(k)Подробнее

How to Prepare a Medical Device 510k Submission for FDA | Rob Packard | Joe HageПодробнее
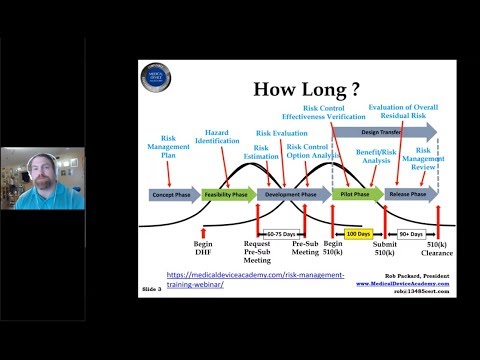
What’s new in the draft FDA Cybersecurity guidance?Подробнее

Webinar: Submission of Cybersecurity Information in Premarket Notification (510k)Подробнее

Learn how to quickly perfect your 510(k) cybersecurity documentation?Подробнее

Using the new eSTAR templates for a 510(k) submission and the FDA eSTAR draft guidanceПодробнее

How to prepare an FDA eSTAR 510(k) submissionПодробнее

Software Validation Documentation for FDA 510(k) pre-market notification submissionПодробнее
