Did you Know? Find 510k by Indications for Use

10 Steps to Preparing Your 510(k) Submission (and How to Avoid the Common Pitfalls)Подробнее

510(k) Project Management - Updated for 2021Подробнее

82 - "What goes into a 510(k)?" - How Do I Get a 510(K) SeriesПодробнее

Mastering your 510(k) submission processПодробнее

De Novo vs 510k - What’s the differenceПодробнее

SARACA Free Live Webinar on Roadblocks in the 510(k) Submission ProcessПодробнее

Before 510k clearance, 10 quality tasks to prevent delays | MDG Premium 077 feat. Rob PackardПодробнее

Webinar: Introduction to US FDA Medical Device Regulations (510k, De Novo, IDE, CAPA, eMDR)Подробнее

Did You Know? Find FDA Clearances by Indications for Use only with Navigator for Medical DevicesПодробнее

Did you Know? Find 510(k) Predicates and Successors in Navigator for Medical DevicesПодробнее

How to Prepare a Medical Device 510k Submission for FDA | Rob Packard | Joe Hage | UpdatedПодробнее
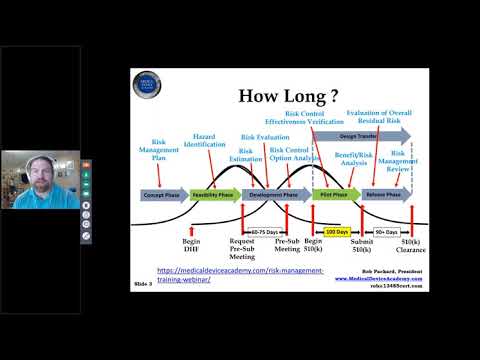
Webinar for Special 510(k) SubmissionsПодробнее
