FDA Regulation of Cardiovascular Devices & UM MICHR's IND/IDE Investigator Assistance Program (MIAP)
FDA Regulation of Cardiovascular Devices & UM MICHR's IND/IDE Investigator Assistance Program (MIAP)Подробнее

FDA Regulation of Medical Devices (Part 1 of 3)Подробнее

FDA Regulation of Medical Devices and Software/AppsПодробнее
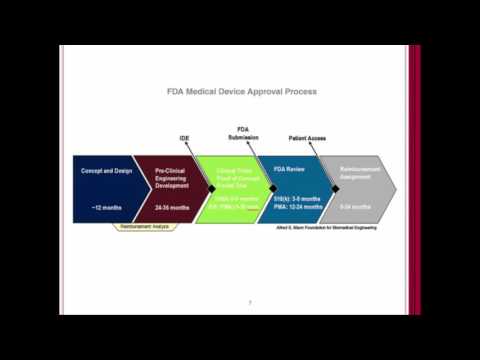
So You Want to Market Your Regulated Digital Health Device in the US?Подробнее

FDA Regulation of Cardiovascular Medical DevicesПодробнее

FDI-IDE Installation GuideПодробнее

FDA Regulation of Medical Devices - Abridged and SimplifiedПодробнее

Webinar: Introduction to US FDA Medical Device Regulations (510k, De Novo, IDE, CAPA, eMDR)Подробнее

Investigational Device ExemptionПодробнее

The 5 most relevant changes the Medical Device Regulation MDR introduces, that you must knowПодробнее

Medical Device Directive (MDD) to Medical Device Regulation (MDR)Подробнее

Regulation of AI-enabled IGT technologies (Health Canada, US FDA, UK MHRA)Подробнее

Medical Device Classification RulesПодробнее

Medical Device Regulations / FDA ApprovalПодробнее

Medical Device Regulation Training - MDSAP TrainingПодробнее

Dmitriy Feldman, MD and Chadi Alraies, MD on aortoiliac disease, approach, and interventionПодробнее

Short course on the Medical Device Regulation (EU) 2017/745Подробнее

Innovator Implications - FDA Regulation of Medical Device Software (Part 3 of 3)Подробнее
