How To Determine The Maximum Number of Electrons Given a Set of Quantum Numbers
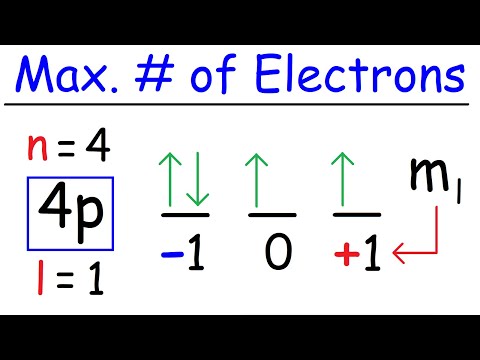
What is the maximum numbers of electrons that can be associated with the following set of quantum...Подробнее

Quantum Numbers - How Many Electrons and Orbitals Have the following set of Quantum Numbers?Подробнее
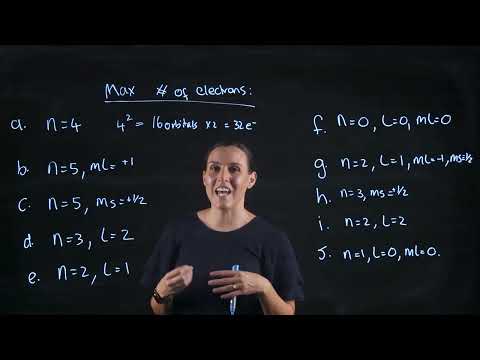
What is the maximum numbers of electrons that can be associated with the following set of quantumПодробнее

Quantum numbers, how to find max number of electrons in a given set? | #5Подробнее

Explain giving reasons, which of the following sets of quantum numbers are not possible?Подробнее

2.3 Electronic Configuration –Electronic Configuration ExamplesПодробнее

MCQ on Quantum numbersПодробнее

How To Calculate The Maximum Number Of Election.(Quantum Numbers)Подробнее

How many electrons are present in a atom having quantum numbers n=3, l=1?Подробнее

The correct set of quantum numbers for 4d-electrons isПодробнее

What is the maximum number of electrons that can be associated with a following set of quantumПодробнее

Orbitals, Quantum Numbers & Electron Configuration - Multiple Choice Practice ProblemsПодробнее

Quantum Numbers - The Easy Way!Подробнее
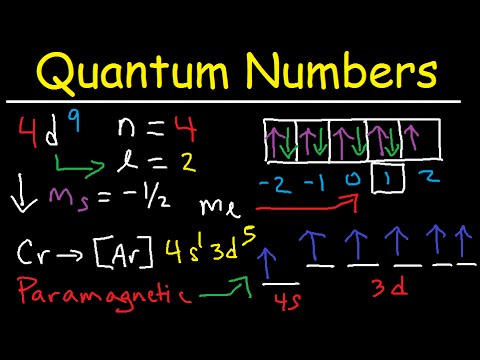
How many electrons in an atom may have the following quantum numbers? a) n=4 m=-1/2 b) n=3 l=0Подробнее

SPDF orbitals Explained - 4 Quantum Numbers, Electron Configuration, & Orbital DiagramsПодробнее
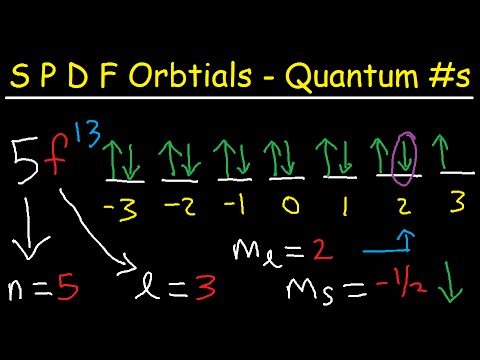
Give the set of quantum number that describe an electron in a `3p` orbital.Подробнее

What is the maximum number of electron in an atom that can have the quantumПодробнее

Quantum Numbers - n, l, ml, ms & SPDF OrbitalsПодробнее
Class 11 Chap 2 | Atomic Structure 05 | Quantam Numbers | Pauli's Exclusion Principle | JEE / NEETПодробнее
